what factors contribute to the rate at which carbon moves between the soil and the atmosphere
*For the new assessment of the concluding decade of understanding the carbon cycle, see the 2d Country of the Carbon Wheel Written report (2018).*
![Net fluxes and transfers of carbon among the atmosphere, land, and water are depicted in this simplified representation of the North American carbon cycle. The diagram includes fluxes of carbon dioxide but not methane or other carbon-containing greenhouse gases. These carbon flows include 1) emissions (red arrows); 2) uptake (black arrows); 3) lateral transfers (blue arrows); and 4) burial (blue arrows), which involves transfers of carbon from water to sediments and soils. Estimates—derived from Figure ES.3 and Figure 2.3 in Ch. 2: The North American Carbon Budget—are in teragrams of carbon (Tg C) per year. The increase in atmospheric carbon, denoted by a positive value, represents the net annual change resulting from the addition of carbon emissions minus net uptake of atmospheric carbon by ecosystems and coastal waters. The estimated increase in atmospheric carbon of +1,009 Tg C per year is from Figure 2.3 and that value is slightly different from the +1,008 Tg C per year value used elsewhere in Ch. 2 because of mathematical rounding. Net ecosystem carbon uptake represents the balance of carbon fluxes between the atmosphere and land (i.e., soils, grasslands, forests, permafrost, and boreal and Arctic ecosystems). Coastal waters include tidal wetlands, estuaries, and the coastal ocean (see Figure ES.3 for details). The net land sink, denoted by a positive value, is the net uptake by ecosystems and tidal wetlands (Figure ES.3) minus emissions from harvested wood and inland waters and estuar- ies (Figure ES.3). For consistency, the land sink estimate of 606 Tg C per year is adopted from Ch. 2. Because of rounding of the numbers in that chapter, this value differs slightly from the combined estimate from Figures ES.2 and ES.3 (605 Tg C per year). Asterisks indicate that there is 95% confidence that the actual value is within 10% (☆☆☆☆☆), 25% (☆☆☆☆), 50% (☆☆☆), 100% (☆☆), or >100% (☆) of the reported value. [Figure source: Adapted from Ciais et al., 2013, Figures 6.1 and 6.2; Copyright IPCC, used with permission.] Source: Second State of the Carbon Cycle Report 2018. Carbon Cycle North America figure from SOCCR2](https://www.carboncyclescience.us/sites/default/files/cciwg/CarbonCycle-North-America-ES_2-1200-SOCCR2.png)
What is the carbon bike? What are the different pools and fluxes of carbon? Why are they important? This page provides a compilation of information and relevant links to assistance answer some of these questions.
The Carbon Cycle:What is the Carbon Cycle? What is the fast and tiresome cycle and how are they influenced?
Carbon Measurement Approaches and Accounting Frameworks: Approaches and methods for carbon stock and menstruum estimations, measurements, and bookkeeping
The North American Carbon Cycle: The latest (2018) assessment and budget
Webinar Serial Videos: 'The State of the Carbon Bicycle: From Science to Solutions'
The Global Carbon Budget : The Global Carbon Upkeep as calculated by a global group of scientists
Frequently asked questions and their answers:Answers to commonly asked questions such as the following are listed hither: Can you quantify the sources and sinks of the global carbon bicycle? How much carbon is stored in the different ecosystems? In terms of mass, how much carbon does ane part per million by volume of atmospheric CO2 represent? What per centum of the COtwo in the temper has been produced by homo beings through the called-for of fossil fuels?
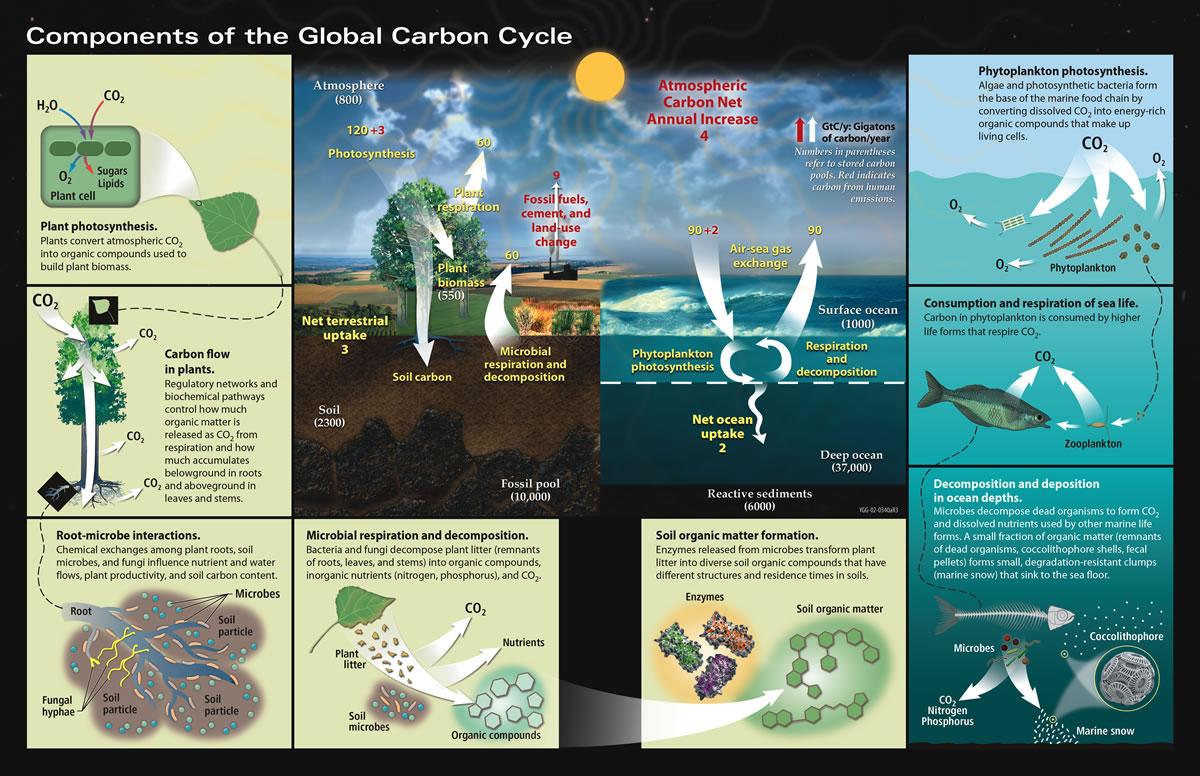
The Carbon Cycle
(Original Source:NASA Earth Observatory)
'Carbon is the backbone of life on Earth. We are made of carbon, we eat carbon, and our civilizations—our economies, our homes, our means of transport—are built on carbon. Nosotros need carbon, but that need is besides entwined with one of the nigh serious problems facing us today: global climate alter.....'
- What is the carbon cycle? 'Carbon flows between each reservoir in an substitution called the carbon cycle, which has slow and fast components. Whatever change in the bicycle that shifts carbon out of ane reservoir puts more carbon in the other reservoirs. Changes that put carbon gases into the temper result in warmer temperatures on Earth....'
- The Slow Carbon Wheel 'Through a series of chemical reactions and tectonic activity, carbon takes between 100-200 meg years to motility between rocks, soil, ocean, and atmosphere in the slow carbon cycle. On average, x13 to ten14 grams (10–100 million metric tons) of carbon move through the slow carbon cycle every year. In comparison, man emissions of carbon to the atmosphere are on the lodge of xfifteen grams, whereas the fast carbon cycle moves 1016 to 1017 grams of carbon per year....'
- The Fast Carbon Cycle: '...Plants and phytoplankton are the chief components of the fast carbon bike. Phytoplankton (microscopic organisms in the body of water) and plants have carbon dioxide from the atmosphere by absorbing it into their cells. Using energy from the Dominicus, both plants and plankton combine carbon dioxide (COtwo) and water to form saccharide (CH2O) and oxygen. The chemical reaction looks like this:
COii + H2O + free energy = CH2O + Otwo
4 things can happen to move carbon from a found and render it to the temper, merely all involve the aforementioned chemic reaction. Plants break downwards the sugar to get the free energy they need to grow. Animals (including people) eat the plants or plankton, and break down the constitute sugar to get energy. Plants and plankton die and decay (are eaten by bacteria) at the end of the growing season. Or burn down consumes plants. In each case, oxygen combines with sugar to release water, carbon dioxide, and energy. The basic chemical reaction looks like this:
CH2O + Oii = CO2 + H2O + energy
In all iv processes, the carbon dioxide released in the reaction commonly ends upward in the atmosphere. The fast carbon bike is so tightly tied to institute life that the growing season can exist seen by the fashion carbon dioxide fluctuates in the atmosphere. In the Northern Hemisphere winter, when few state plants are growing and many are decaying, atmospheric carbon dioxide concentrations climb. During the spring, when plants begin growing once more, concentrations drop. It is as if the Globe is breathing. The ebb and period of the fast carbon cycle is visible in the changing seasons. Equally the big land masses of Northern Hemisphere dark-green in the bound and summer, they draw carbon out of the temper. This graph shows the difference in carbon dioxide levels from the previous calendar month, with the long-term trend removed. This cycle peaks in August, with about 2 parts per one thousand thousand of carbon dioxide drawn out of the atmosphere. In the autumn and winter, every bit vegetation dies back in the northern hemisphere, decomposition and respiration returns carbon dioxide to the atmosphere. These maps bear witness net primary productivity (the amount of carbon consumed by plants) on land (light-green) and in the oceans (bluish) during August and December, 2010. In August, the green areas of N America, Europe, and Asia represent plants using carbon from the atmosphere to grow. In December, net primary productivity at high latitudes is negative, which outweighs the seasonal increase in vegetation in the southern hemisphere. Every bit a result, the amount of carbon dioxide in the atmosphere increases....'
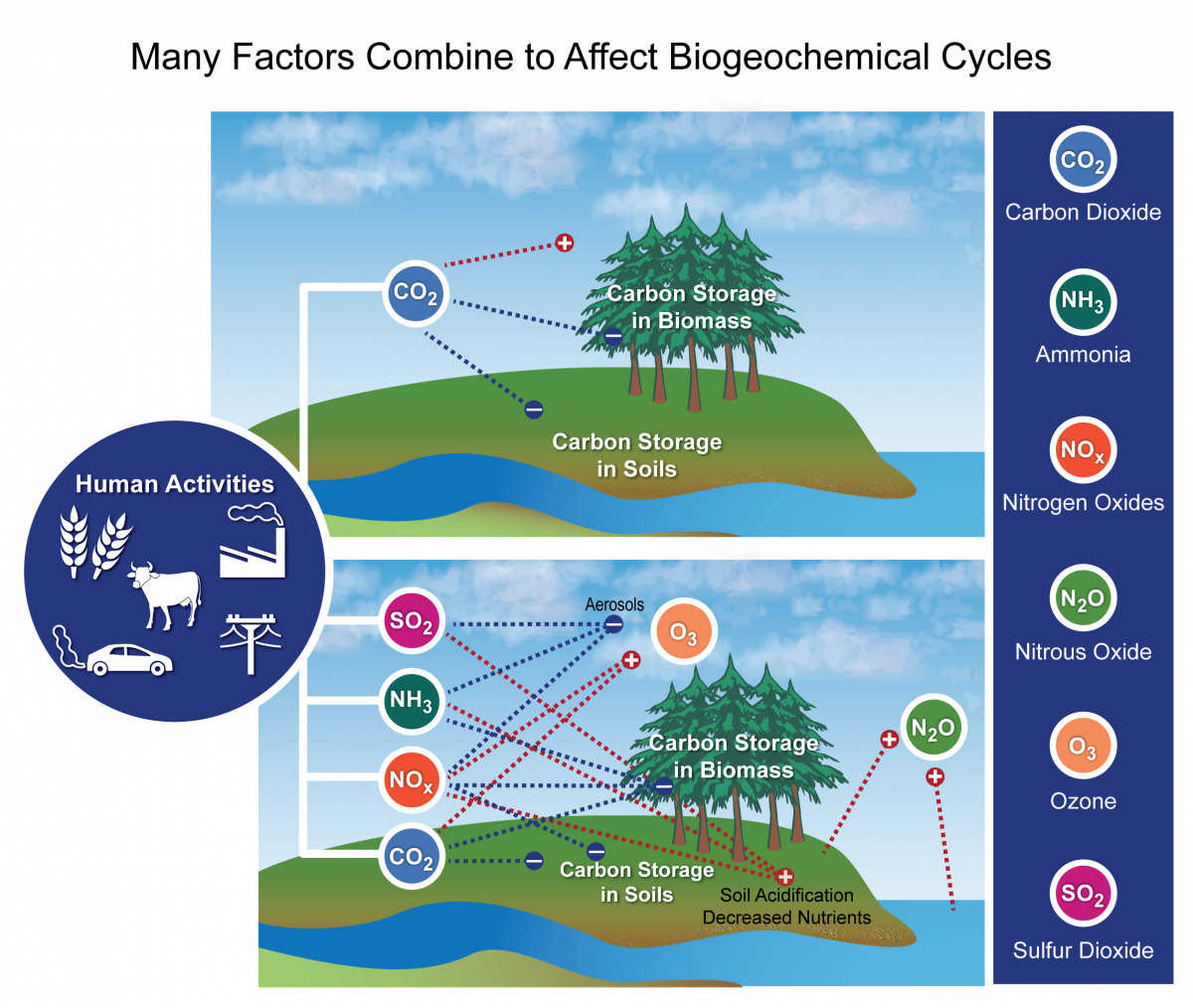
- Changes in the Carbon Wheel 'Left unperturbed, the fast and ho-hum carbon cycles maintain a relatively steady concentration of carbon in the temper, land, plants, and sea. Simply when anything changes the corporeality of carbon in one reservoir, the effect ripples through the others....'
- Effects of Changing the Carbon Bike 'It is significant that so much carbon dioxide stays in the atmosphere because COii is the virtually important gas for decision-making Earth'due south temperature. Carbon dioxide, methane, and halocarbons are greenhouse gases that absorb a wide range of energy—including infrared energy (heat) emitted by the Globe—so re-emit information technology. The re-emitted energy travels out in all directions, only some returns to World, where information technology heats the surface. Without greenhouse gases, Earth would exist a frozen -eighteen degrees Celsius (0 degrees Fahrenheit). With too many greenhouse gases, Earth would be like Venus, where the greenhouse atmosphere keeps temperatures around 400 degrees Celsius (750 Fahrenheit)....'
- Studying the Carbon Bike 'What volition those changes look like? What volition happen to plants as temperatures increment and climate changes? Will they remove more than carbon from the atmosphere than they put back? Will they get less productive? How much extra carbon will melting permafrost put into the atmosphere, and how much will that dilate warming? Will ocean circulation or warming change the rate at which the ocean takes up carbon? Will ocean life become less productive? How much will the ocean acidify, and what furnishings volition that have?....' (Original Source: NASA Earth Observatory)
Carbon measurement Approaches and Accounting Frameworks
From the State of the Carbon Cycle Report (USGCRP, 2018) Preface (Shrestha et al, 2018):
'Three observational, analytical, and modeling methods are used to estimate carbon stocks and fluxes: one) inventory measurements or "bottom-up" methods, ii) atmospheric measurements or "top-down" methods, and 3) ecosystem models (see Appendix D for details). "Bottom-up" estimates of carbon exchange with the atmosphere depend on measurements of carbon independent in biomass, soils, and water, likewise as measurements of CO2 and CH4 exchange among the country, h2o, and atmosphere. Examples include straight measurement of power plant carbon emissions; remote-sensing and field measurements repeated over fourth dimension to judge changes in ecosystem stocks; measurements of the amount of carbon gases emitted from state and water ecosystems to the atmosphere (in chambers or, at larger scales, using sensors on towers); and combined urban demographic and activity data (e.thousand., population and edifice floor areas) with "emissions factors" to estimate the amount of CO2 released per unit of measurement of activity.
Top-down approaches infer fluxes from the terrestrial country surface and sea by coupling atmospheric gas measurements (using air sampling instruments on the footing, towers, buildings, balloons, and aircraft or remote sensors on satellites) with carbon isotope methods, tracer techniques, and simulations of how these gases move in the atmosphere. The network of GHG measurements, types of measurement techniques, and diversity of gases measured has grown exponentially since SOCCR1 (CCSP 2007), providing improved estimates of COii and CHiv emissions and increased temporal resolution at regional to local scales beyond North America.
Ecosystem models are used to estimate carbon stocks and fluxes with mathematical representations of essential processes, such equally photosynthesis and respiration, and how these processes respond to external factors, such equally temperature, precipitation, solar radiations, and h2o motion. Models also are used with top-down atmospheric measurements to aspect observed GHG fluxes to specific terrestrial or sea features or locations.'
For details, see the SOCCR2 Preface (Shrestha et al. 2018) and Appendix D (Birdsey et al. 2018).
References:
Shrestha, Chiliad., Due north. Cavallaro, R. Birdsey, M. A. Mayes, R. G. Najjar, S. C. Reed, P. Romero-Lankao, N. P. Gurwick, P. J. Marcotullio, and J. Field, 2018: Preface. In Second Country of the Carbon Cycle Report (SOCCR2): A Sustained Assessment Report [Cavallaro, N., G. Shrestha, R. Birdsey, M. A. Mayes, R. G. Najjar, Southward. C. Reed, P. Romero-Lankao, and Z. Zhu (eds.)]. U.S. Global Change Enquiry Program, Washington, DC, USA, pp. 5-20, https://doi.org/x.7930/SOCCR2.2018.Preface.
Birdsey, R., N. P. Gurwick, K. R. Gurney, One thousand. Shrestha, K. A. Mayes, R. Chiliad. Najjar, Southward. C. Reed, and P. RomeroLankao, 2018: Appendix D. Carbon measurement approaches and bookkeeping frameworks. In 2nd State of the Carbon Cycle Study (SOCCR2): A Sustained Cess Study [Cavallaro, Due north., Grand. Shrestha, R. Birdsey, M. A. Mayes, R. G. Najjar, S. C. Reed, P. Romero-Lankao, and Z. Zhu (eds.)]. U.Due south. Global Change Enquiry Plan, Washington, DC, Usa, pp. 834-838, doi: https:// doi.org/10.7930/SOCCR2.2018.AppD.
Back to acme
The Northward American Carbon Bicycle and Budget
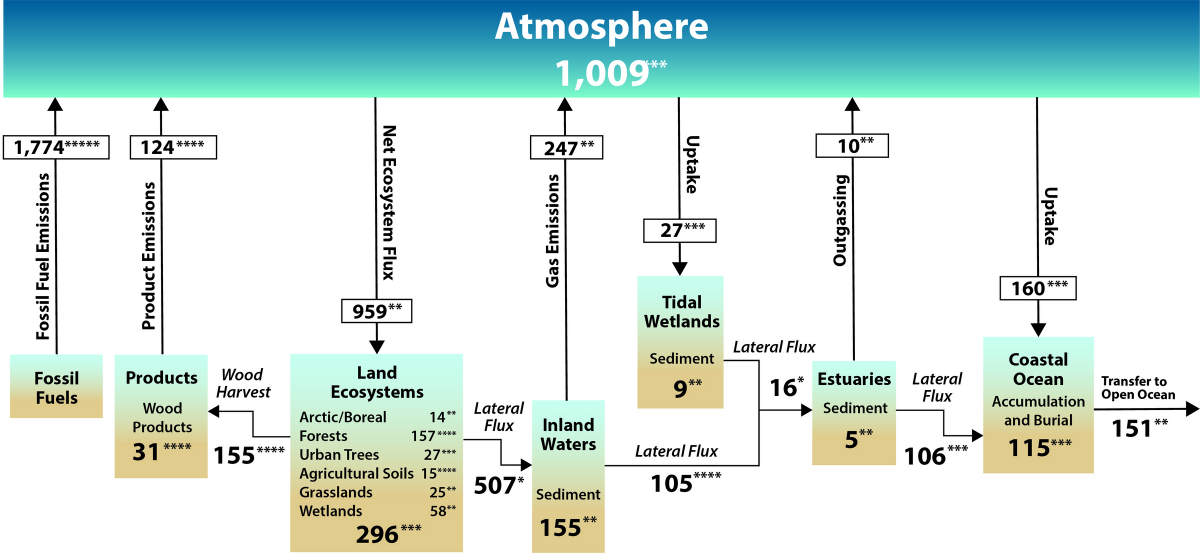
Excerpt from the Second Land of the Carbon Wheel Written report (SOCCR2, USGCRP 2018) Chapter 2 (Hayes et. al, 2018):
'Since the Industrial Revolution, human activity has released into the atmosphere unprecedented amounts of carbon-containing greenhouse gases (GHGs), such as carbon dioxide (CO2) and methane (CHfour), that take influenced the global carbon cycle. For the past three centuries, N America has been recognized as a net source of CO2 emissions to the temper (Houghton 1999, 2003; Houghton and Hackler 2000; Hurtt et al., 2002). Now there is greater interest in including in this film emissions of CHiv because it has 28 times the global warming potential of CO2 over a 100-year time horizon (Myhre et al., 2013; NAS 2018).
The major continental sources of CO2 and CH4 are ane) fossil fuel emissions, 2) wildfire and other disturbances, and three) land-utilize modify. Globally, continental carbon sources are partially offset past sinks from natural and managed ecosystems via establish photosynthesis that converts CO2 into biomass. The terrestrial carbon sink in North America is known to kickoff a substantial proportion of the continent's cumulative carbon sources. Although uncertain, quantitative estimates of this kickoff over the last two decades range from equally low as xvi% to as loftier as 52% (King et al., 2015). Highlighted in this chapter are persistent challenges in unravelling CH4 dynamics across North America that arise from the need to fully quantify multiple sources and sinks, both natural (Warner et al., 2017) and anthropogenic (Hendrick et al., 2016; Turner et al., 2016a; NAS 2018). Calculation to the challenge is disagreement on whether the reported magnitudes of CH4 sources and sinks in the United States are underestimated (Bruhwiler et al., 2017; Miller et al., 2013; Turner et al., 2016a).
At the global scale, most 50% of annual anthropogenic carbon emissions are sequestered in marine and terrestrial ecosystems (Le Quéré et al., 2016). Temporal patterns indicate that fossil carbon emissions have increased from three.three petagrams of carbon (Pg C) per year to almost 10 Pg C over the past l years (Le Quéré et al., 2015). Still, considerable uncertainty remains in the spatial patterns of emissions at finer scales over which carbon management decisions are fabricated. Most importantly, the sensitivity of terrestrial sources and sinks to variability and trends in the biophysical factors driving the carbon cycle is not understood well enough to provide practiced confidence in projections of the hereafter performance of the North American carbon balance (Friedlingstein et al., 2006; McGuire et al., 2016; Tian et al., 2016).'
For further details, meet the latest decadal assessment of North American Carbon Cyle, the Second State of the Carbon Wheel Report.
References:
Hayes, D. J., R. Vargas, South. R. Alin, R. T. Conant, Fifty. R. Hutyra, A. R. Jacobson, Westward. A. Kurz, S. Liu, A. D. McGuire, B. Poulter, and C. W. Woodall, 2018: Chapter ii: The North American carbon budget. In Second State of the Carbon Cycle Study (SOCCR2): A Sustained Cess Report [Cavallaro, Due north., G. Shrestha, R. Birdsey, G. A. Mayes, R. G. Najjar, S. C. Reed, P. Romero-Lankao, and Z. Zhu (eds.)]. U.S. Global Change Research Plan, Washington, DC, The states, pp. 71-108, https://doi.org/10.7930/SOCCR2.2018.Ch2.
USGCRP, 2018: Second State of the Carbon Cycle Report (SOCCR2): A Sustained Assessment Study. [Cavallaro, N., G. Shrestha, R. Birdsey, Thou. A. Mayes, R. G. Najjar, S. C. Reed, P. Romero-Lankao, and Z. Zhu (eds.)]. U.South. Global Change Research Program, Washington, DC, U.s., 878 pp., https://doi.org/10.7930/SOCCR2.2018
Back to peak
Webinar Series Videos: 'The Country of the Carbon Cycle: From Science to Solutions' and others
Recorded webinars describing what is the carbon cycle, focusing on the 2nd Country of the Carbon Wheel Report science findings and pertinent scientific and societally-relevant activities, are posted on our YouTube Channel. The serial desciption is here.
Dorsum to tiptop
Global carbon budget
Note: For the latest annual global carbon and methyl hydride budgets, please see the Global Carbon Project.
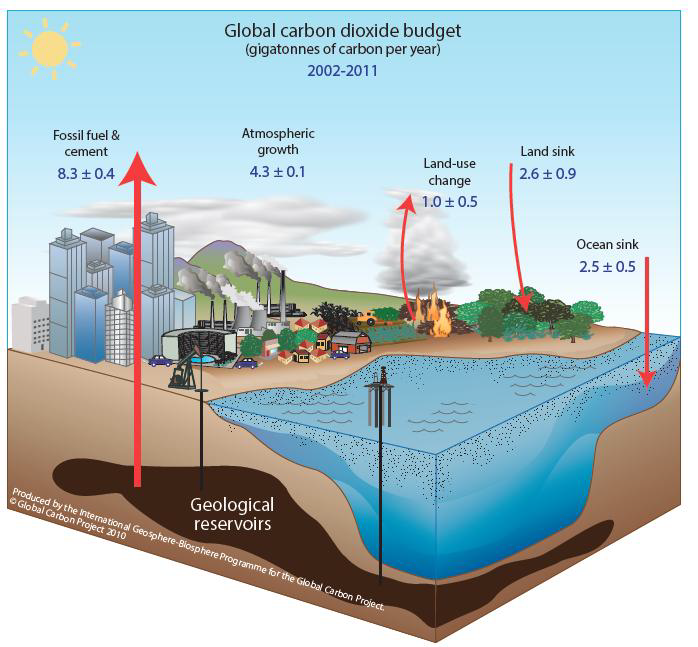
The adjacent figure on the left represents recent global carbon budget estimates of annual carbon flows averaged from 2002 to 2011 , every bit provided in the Global Carbon Projection'south 2013 report. (Values in gigatons of carbon per yr)
Note:
1 GtC = 1 gigaton of carbon (or British-French 1gigatonne of carbon)
= ten 9 metric ton carbon or i billion tons of carbon
= 1 PgC = 1 petagram of carbon = 10 15 chiliad of carbon
1 metric ton = thousand Kg = 10 6 thou
(The metric ton is too written equally tonne in the British and French systems, equally in this Global Carbon Upkeep figure.)
Back to elevation
Frequently asked questions and their answers most the carbon cycle
(Source: Carbon Dioxide Information Assay Heart, CDIAC)
Q. What are the present tropospheric concentrations, global warming potentials (100 year fourth dimension horizon), and atmospheric lifetimes of CO 2 , CH four , Due north 2 O, Cfc-xi, CFC-12, Chlorofluorocarbon-113, CCl 4 , methyl chloroform, HCFC-22, sulphur hexafluoride, trifluoromethyl sulphur pentafluoride, perfluoroethane, and surface ozone?
A. View a table presenting information and source for current greenhouse gas concentrations.
Q. Tin can you quantify the sources and sinks of the global carbon cycle?
A. Read a give-and-take of the global carbon cycle. You lot may besides view the figures here (adapted by CDIAC from the IPCC 4th Cess Report: Climatic change 2007 and the Woods Hole Research Center.)
Notation: GtC = gigaton of carbon and giga = 109 and Pg C = petagram of carbon and Peta = x15
Detect the latest carbon upkeep estimates. Source: Global Carbon Project
And, click here to run into figures summarizing the global cycles of biologically active elements. Source: William Southward. Reeburgh, Professor Marine and Terrestrial Biogeochemistry, University of California.
Q. How much carbon is stored in the different ecosystems?
A. View an illustration of the major world ecosystem complexes ranked by carbon in live vegetation.
Q. In terms of mass, how much carbon does 1 role per million by volume of atmospheric CO ii correspond?
A. Using 5.137 x x xviii kg as the mass of the atmosphere (Trenberth, 1981 JGR 86:5238-46), 1 ppmv of CO two = 2.13 Gt of carbon.
Back to meridian
Q. What percentage of the CO ii in the atmosphere has been produced by human being beings through the burning of fossil fuels?
A. Anthropogenic CO 2 comes from fossil fuel combustion, changes in land utilize (e.g., forest clearing), and cement manufacture. Houghton and Hackler take estimated land-employ changes from 1850-2000, and then it is user-friendly to apply 1850 equally our starting bespeak for the post-obit give-and-take. Atmospheric CO 2 concentrations had not changed appreciably over the preceding 850 years (IPCC; The Scientific Footing) so it may be safely causeless that they would not have changed appreciably in the 150 years from 1850 to 2000 in the absenteeism of human intervention.
In the post-obit calculations, nosotros will express atmospheric concentrations of CO 2 in units of parts per million by volume (ppmv). Each ppmv represents 2.13 X10 xv grams, or two.13 petagrams of carbon (PgC) in the atmosphere. According to Houghton and Hackler, land-use changes from 1850-2000 resulted in a internet transfer of 154 PgC to the atmosphere. During that aforementioned period, 282 PgC were released past combustion of fossil fuels, and 5.5 boosted PgC were released to the atmosphere from cement manufacture. This adds upwards to 154 + 282 + 5.5 = 441.5 PgC, of which 282/444.1 = 64% is due to fossil-fuel combustion.
Atmospheric CO 2 concentrations rose from 288 ppmv in 1850 to 369.5 ppmv in 2000, for an increase of 81.v ppmv, or 174 PgC. In other words, about twoscore% (174/441.5) of the additional carbon has remained in the atmosphere, while the remaining 60% has been transferred to the oceans and terrestrial biosphere.
The 369.5 ppmv of carbon in the atmosphere, in the form of CO two , translates into 787 PgC, of which 174 PgC has been added since 1850. From the second paragraph to a higher place, nosotros run into that 64% of that 174 PgC, or 111 PgC, tin be attributed to fossil-fuel combustion. This represents about 14% (111/787) of the carbon in the atmosphere in the class of CO 2 .
Meet the lastest Country of the Carbon Cycle Written report for details.
Back to top
Q. How much carbon dioxide is produced from the combustion of 1000 cubic feet of natural gas?
A. If we start with 1000 cubic feet of natural gas (and assuming it is pure methane or CH four ) at STP (standard temperature and pressure level, i.due east., temperature of 273°K = 0°C = 32°F and pressure of i atm = 14.7 psia = 760 torr), and burn it completely, here's what we come up with:
1 cubic pes (cf) = 0.0283165 cubic meters (m 3 )
and 1 thou three = 1000 liters (50)
so 1 cf = 28.31685 L
and 1000 cf = 28316.85 L
Since one mole of a gas occupies 22.4 L at STP, 28316.85 Fifty of CH 4 contains 28316.85/22.4 = 1264.145 moles of CH 4 (each mole of CH 4 = approx. xvi thousand)
If we burn down CH four completely, information technology follows this equation:
CH four + 2O two => CO 2 + 2H ii 0
That is, for each mole of methane we get i mole of carbon dioxide.
1 mole of CO 2 has a mass of approx. 44 thou, so 1264.145 moles of CO 2 has a mass of approx. 1264.145 x 44 or 55622.38 g
A pound is nearly equivalent to 454 chiliad, so 55622.38 1000 is nearly equivalent to 55622.38/454 or 122 lb
That is, the complete combustion of chiliad cubic feet at STP of natural gas results in the production of about 122 lb of carbon dioxide.
Of grade, the mass of the methane in 1000 cubic anxiety volition vary if the temperature and pressure are Non equally assumed above, and this will bear on the mass of CO2 produced. According to the Ideal Gas Law:
PV = nRT
Where P = pressure
5 = book
northward = moles of gas
T = temperature
R = constant (0.08206 50 atm/mole K or 62.36 L torr/mole K)
At STP, 1000 cf contains
northward = PV/RT moles of methane
= (1 atm)(28316.85 L)/(0.08206 L atm/mole K)(273°K)
= 1264 moles CH4 (the value given in the instance above)
In the energy industry, however, i standard cubic pes (scf) of natural gas is defined at 60°F (= 15.6°C = 288.6°K) and 14.seven psia, rather than at STP (Handbook of Formulae, Equations and Conversion Factors for the Energy Professional, Task Publications, Tallahassee, FL;). Solving again at this higher (relative to STP) temperature, nosotros get:
northward = (1 atm)(28316.85 L)/(0.08206 L atm/mole K)(288.6°K) = 1196 moles CH4
That is, at the higher temperature, a given volume of gas will contain fewer moles, and less mass. Going again through the adding for CO 2 emitted, only using the value of 1196 moles of CH 4 , results in an reply of approximately 115 lb of carbon dioxide.
Dorsum to top
Q. Why exercise some estimates of CO 2 emissions seem to exist near 3 1/2 times equally big as others?
A. When looking at CO ii emissions estimates, it is important to look at the units in which they are expressed. The numbers are sometimes expressed as mass of CO 2 but are listed in all of our estimates only in terms of the mass of the C (carbon). Because C cycles through the temper, oceans, plants, fuels, etc. and changes the ways in which information technology is combined with other elements, it is often easier to go on rails but of the flows of carbon. Emissions expressed in units of C tin can exist easily converted to emissions in CO 2 units by adjusting for the mass of the fastened oxygen atoms, that is by multiplying past the ratios of the molecular weights, 44/12, or 3.67.
Q. Why is the sum of all national and regional CO 2 emission estimates less than the global totals?
A. The difference between the sum of the private countries (or regions) and the global estimates is generally less than v%. There are four primary reasons for this.
- global totals include emissions from bunker fuels whereas these are not included in national (or regional) totals. Bunker fuels are fuels used by ships and aircraft in international transportation,
- global totals include estimates for the oxidation of non-fuel hydrocarbon products (due east.thousand., asphalt, lubricants, petroleum waxes, etc.) whereas national totals do not,
- national totals include annual changes in fuel stocks whereas the global total does non, and
- due to statistical differences in the international statistics, the sum of exports from all exporters is non identical to the sum of all imports by all importers.
Q. Why practise some smaller nations have larger per capita emission estimates than industrialized nations like the Usa?
A. Often it is difficult to aspect emissions to a source. Many small island nations have armed forces bases that are used for re-fueling or have large tourist industries. Who do you assign the emissions to; the The states whose war machine planes are re-fueling on the Wake Island with aviation and jet fuel or the Wake Island? The bookkeeping practices used within the UN Energy Statistics Database assign this fuel consumption to the Wake Island thus elevating the Wake Island'due south per capita gauge. The same is true for tourist nations like Aruba who are assigned the fuels used in the commercial planes conveying tourists back to their native countries. Although this distorts the per capita emission estimates information technology makes it easier from an bookkeeping standpoint than trying to trace each plane or ship to its final destination. One should be cautious in using only the per capita CO 2 emission estimates.
Back to top
Q. What is the greenhouse effect? Is it the same as the ozone pigsty issue?
A. No, they are two dissimilar (only related) issues.The greenhouse effect effect concerns the warming of the lower function of the atmosphere, the troposphere (the layer in which temperature drops with height; it is about x-15 kilometers thick, varying with latitude and season), by increasing concentrations of the so-called greenhouse gases (carbon dioxide, methane, nitrous oxide, ozone, and others) in the troposphere. This warming occurs considering the greenhouse gases, while they are transparent to incoming solar radiation, absorb infrared (rut) radiation from the Earth that would otherwise escape from the atmosphere into space; the greenhouse gases and so re-radiate some of this heat back towards the surface of the Globe.
The ozone hole effect concerns the loss of ozone in the upper office of the atmosphere, the stratosphere, resulting from increasing concentrations of certain halogenated hydrocarbons (such as chlorinated fluorocarbons, known as CFCs). Through a series of chemical reactions in the stratosphere, the halogenated hydrocarbons destroy ozone in the stratosphere. This is of business organisation considering the ozone blocks incoming ultraviolet radiation from the Sun, and portions of the ultraviolet radiation spectrum have been found to have adverse biological effects.
The greenhouse effect and ozone pigsty problems are, notwithstanding, related. For example, CFCs are involved in both problems: CFCs, in addition to destroying stratosphere ozone, are also greenhouse gases. Information technology has traditionally been thought there is not much mixing of the troposphere and stratosphere. But there is recent evidence of transport of stratospheric ozone into the troposphere (see "Ozone-rich transients in the upper equatorial Atlantic troposphere," by Suhre et al., Nature , Vol. 388, xiv Baronial 1997, pages 661-663, and the related discussion paper, "Ozone clouds over the Atlantic," past Crutzen and Lawrence, on pages 625-626 in the same issue of Nature ). And then ozone depletion in the stratosphere could result in reduced concentrations of this greenhouse gas in the troposphere. Conversely, global climate change could also touch ozone depletion through changes in stratospheric temperature and water vapor (see "The event of climate change on ozone depletion through changes in stratospheric h2o vapour," past Kirk-Davidoff et al., Nature, Vol. 402, 25 Nov 1999, pages 399-401). [RMC]
Q. Should we be concerned with human being animate as a source of CO 2 ?
A. No. While people exercise exhale carbon dioxide (the rate is approximately 1 kg per day, and it depends strongly on the person'southward activity level), this carbon dioxide includes carbon that was originally taken out of the carbon dioxide in the air past plants through photosynthesis - whether y'all swallow the plants directly or animals that eat the plants. Thus, in that location is a airtight loop, with no net addition to the atmosphere. Of course, the agronomics, food processing, and marketing industries use free energy (in many cases based on the combustion of fossil fuels), but their emissions of carbon dioxide are captured in our estimates as emissions from solid, liquid, or gaseous fuels.
Back to pinnacle
Q. How does the oxygen wheel relate to the greenhouse consequence and global warming?
A. With recent developments information technology is now feasible to measure variations in the oxygen content of the temper at the parts per million (ppm) level. Regular measurements of changes in atmospheric oxygen (O 2 ) are currently being made at a number of locations around the world using two independent techniques, 1 based on interferometry and ane based on stable isotope mass spectroscopy. Oxygen measurements can inform u.s.a. about cardinal aspects of the global carbon bike. Oxygen is generated past green plants in photosynthesis and converted to carbon dioxide (CO 2 ) in beast and human respiration. Carbon dioxide is the greenhouse gas of most business organization due to its affluence in the atmosphere (~ 360 ppm) and anthropogenic sources. Variations in atmospheric O two are controlled largely by fluxes of carbon (eastward.g., photosynthesis and respiration CO 2 + H two O <=> CH 2 O + O 2 ).
For farther reading, we suggest:
Keeling, R.F., D.A. Najjar, M.L. Bender, P.P. Tans. 1993. What Atmospheric Oxygen Measurements Can Tell Us About The Global Carbon Cycle. Global Biogeochemical Cycles vii:37-67.
Moore, B. Iii, and B.H. Braswell. 1994. The lifetime of excess atmospheric carbon dioxide. Global Biogeochemical Cycles 8:23-38.
Keeling, R.F. and S.R. Shertz. 1992. Seasonal and interannual variations in atmospheric oxygen and implications for the global carbon bicycle. Nature 358:723-727.
Broecker, W. and J.P.Severinghaus. 1992. Diminishing Oxygen. Nature 358:710-711. Back to top
Q. How long does it take for the oceans and terrestrial biosphere to have upwardly carbon later on information technology is burned?
A. With over 800 billion metric tons of carbon in the atmosphere and an annual exchange with the biosphere and oceans equal to around 200 billion metric tons, an boilerplate atom of carbon spends only about 4 years in the temper earlier it goes into the oceans or the terrestrial biosphere. We can remember of this equally the average residence time for a carbon atom in the temper. Yet, the oceans and terrestrial biosphere non only take up carbon from the atmosphere (east.1000., absorption by the oceans and photosynthesis past plants) but they too give information technology back (eastward.g., emission from oceans and respiration by animals). That is, nearly of these carbon atoms are "recycled" then the atmosphere is not entirely rid of them. The time it takes for a carbon cantlet to make information technology out of this recycling organisation and to get into the deep ocean is about 100 years. The effigy below, provided by Ken Caldiera of the Carnegie Establishment for Scientific discipline, shows how an instantaneous doubling of pre-industrial carbon dioxide (from 280 parts per million to 560 parts per million) would be removed from the atmosphere-biosphere organisation. Nigh 50% of the added COtwo would be removed after about 200 years and well-nigh 80% of it would exist removed after almost 1000 years, simply consummate removal of the remaining 20% to the deep ocean and carbonate rocks would have to rely on geological processes operating over much longer fourth dimension periods.
Q. How much CO 2 is emitted as a result of my using specific electrical appliances?
A. For this answer, we refer you lot to an fantabulous article, "Your Contribution to Global Warming," by George Barnwell, which appeared on p. 53 of the Feb-March 1990 result of National Wildlife, the mag of the National Wildlife Federation. The article, assuming that your electricity comes from coal, calculates CO 2 emissions corresponding to the use of various electric appliances. For example, i hr's utilize of a color television produces 0.64 pounds (lb) of CO 2 , and each use of a toaster produces 0.12 lbCO 2 , whereas a twenty-four hour period's use of a waterbed heater produces 24 lb CO two .
In general, the coefficient is about 2.3 lb CO 2 per kilowatt-hr (kWh) of electricity. You can calculate the kWh of electricity by multiplying the number of watts (Due west) the appliance uses times the number of hours (h) it is used, then dividing by grand. For example a 60-West light seedling operated for 24 h uses (60 W) 10 (24 h) / (1000) = i.44 kWh.
This use of electricity would produce an emission of (one.44 kWh) x (2.three lb CO 2 per kWh) = 3.3 lb CO 2 if the electricity is derived from the combustion of coal.
Q. Why do certain compounds, such equally carbon dioxide, absorb and emit infrared energy?
A. Molecules can absorb and emit 3 kinds of free energy: free energy from the excitation of electrons, energy from rotational motion, and free energy from vibrational motion. The offset kind of energy is also exhibited by atoms, but the second and third are restricted to molecules. A molecule can rotate about its center of gravity (there are three mutually perpendicular axes through the center of gravity). Vibrational energy is gained and lost as the bonds between atoms, which may be idea of equally springs, expand and contract and bend. The iii kinds of energy are associated with different portions of the spectrum: electronic energy is typically in the visible and ultraviolet portions of the spectrum (for case, wavelength of ane micrometer, vibrational energy in the most infrared and infrared (for instance, wavelength of iii micrometers), and rotational energy in the far infrared to microwave (for case, wavelength of 100 micrometers). The specific wavelength of absorption and emission depends on the type of bond and the type of group of atoms within a molecule. Thus, the stretching of the C-H bail in the CH 2 and CH 3 groups involves infrared energy with a wavelength of 3.3-3.4 micrometers. What makes certain gases, such as carbon dioxide, act as "greenhouse" gases is that they happen to have vibrational modes that absorb energy in the infrared wavelengths at which the earth radiates energy to space. In fact, the measured "peaks" of infrared absorbance are ofttimes broadened because of the overlap of several electronic, rotational, and vibrational energies from the several-to-many atoms and interatomic bonds in the molecules. (Information from "Basic Principles of Chemistry" by Harry B. Gray and Gilbert P. Haight, Jr., published 1967 past W. A. Benjamin, Inc., New York and Amsterdam)
Q. Is it possible to separate the carbon and oxygen from CO 2 every bit is possible with other molecules?
A. The trouble in separating the carbon and oxygen from CO ii is that CO 2 is a VERY stable molecule, considering of the bonds that hold the carbon and oxygen together, and it takes a lot of energy to separate them. Well-nigh schemes beingness considered now involve conversion to liquid or solids. One present concept for capturing CO 2 , such every bit from flue gases of boilers, involves chemical reaction with MEA (monoethanol amine). Other techniques include physical absorption; chemic reaction to methanol, polymers and copolymers, effluvious carboxylic acid, or urea; and reaction in constitute photosynthetic systems (or synthetic versions thereof). Overcoming energetic hurdles is a major claiming; if the energy needed to drive these reactions comes from called-for of fossil fuels, there may not exist an overall gain. One attribute of the current research is the use of catalysts to promote the reactions. (In green plants, of course, chlorophyll is such a catalyst!) One area of current research is looking at using cellular components to imitate photosynthesis on an industrial scale. For example, run into http://www.ornl.gov/ornl94/looking.html which describes inquiry of the Chemic Applied science Division at Oak Ridge National Laboratory.
The International Energy Bureau'due south Greenhouse Gas R&D Plan has many activities in the area of separation and sequestration of CO 2 - see their web site (http://www.ieagreen.org.uk/). [RMC]
Back to top
Q. I am curious about the global warming potential of water vapor. Practise you lot know if estimates are washed of this in the same way as global warming potentials are calculated for other greenhouse gases? I am also interested in why no mention is always fabricated of the enhanced greenhouse consequence acquired by anthropogenic emissions of water vapor. Are the anthropogenic emissions not pregnant?
A. H2o vapor is indeed a very potent "greenhouse" gas, in terms of its arresting and re-radiating outgoing infrared radiation. It is commonly not mentioned as an important factor in global warming, because it is not clear that the atmospheric concentration (as compared with CO two , methane, etc.) is rising. Some (Richard Lindzen at MIT, prominently) have argued that the uncertain potential feedbacks involving water vapor represent a serious shortcoming in models of climate warming. See the following online resources for a good word of this result:
http://www.eia.doe.gov/cneaf/pubs_html/attf94_v2/chap2.html
Q. Is there ecology touch on/business organisation (greenhouse emissions) associated with technologies using CO two (east.g. dry ice blasting, supercritical cleaning, painting, etc.,). If then, are there electric current or impending regulation specific to their use?
A. "Nigh of the CO two used in these kinds of applications is recovered from processes like fermentation and it is either CO 2 that it is being extracted from the atmosphere by plants or CO ii that would have been released from fossil fuel called-for anyway. In essence it passes through this kind of use rather than being emitted immediately and at that place is no actress CO 2 produced".
Q. Could y'all tell me, please, if I have 1 gallon of fuel in my car, how many (units?) of CO 2 volition be emitted? Is in that location any divergence if the machine 4 or 6 or 8 cylinders or in respect of horse power in pct?
A. A good estimate is that y'all volition discharge 19.6 pounds of CO 2 from burning 1 gallon of gasoline. This does not depend on the power or configuration of the engine but depends only on the chemical science of the fuel. Of course if the car gets more miles per gallon of gasoline, you will go less CO 2 per unit of measurement of service rendered (that is, less CO two per mile traveled).[GM]
The U.Southward. Department of Free energy and the U.S. Environmental Protection Bureau recently launched a new Fuel Economy Web Site designed to help the public factor energy efficiency into their car buying decisions. This site offers data on the connection between fuel economic system, avant-garde technology, and the environment.
Q. How much CO 2 do you become from combustion of fossil fuels? How tin can the mass of the CO 2 be greater than the mass of the fuel burned?
A. Let us illustrate with the combustion of natural gas (methane).
C = carbon, atomic weight approximately 12
H = hydrogen, atomic weight approximately one
O = oxygen, atomic weight approximately sixteen
CH 4 = methane, molecular weight approximately 16
O 2 = molecular oxygen, molecular weight approximately 32
CO 2 = carbon dioxide, molecular weight approximately 44
H ii O = water, molecular weight approximately 18
For combustion of methane
CH iv + 2O 2 = CO 2 + 2H two O
So, combustion of 16 mass units (grams, pounds, whatever) of methane produces 44 mass units of carbon dioxide and 36 mass units of h2o while consuming 64 mass units of oxygen. [GM]
Back to top
Q. Is at that place any ONE person who discovered global warming? If not, what year was global warming discovered?.
A. The commencement person to have predicted that emissions of carbon dioxide from the called-for of fossil fuels would crusade a global warming is considered to be S. Arrhenius, who published in 1896 the paper "On the influence of carbonic acrid in the air upon the temperature of the ground." That atmospheric carbon dioxide was actually increasing was confirmed start in the 1930s, and convincingly so beginning in the late 1950s when highly authentic measurement techniques were developed (the most famous sit-in of this is in C.D. Keeling's tape at Mauna Loa, Hawaii). By the 1990s, it was widely accustomed (only not unanimously and so) that the World's surface air temperature had warmed over the by century. An ongoing debate is whether such a warming tin, in fact, exist attributed to increasing carbon dioxide in the atmosphere.
Q. Where may I obtain data on the backdrop of CO 2 ?
A. National Institute of Standards and Engineering science's spider web site
Q. I would like to know whether or non significant amounts of soil organic matter (SOM) and freshly fallen litter in a forest ecosystem tin can be degraded ABIOTICALLY (i.e., through chemical or physical processes) and consequently generate CO two . In other words, can I consider that entire CO 2 emitted from soil is derived from biological processes?
A. Earliest quantitative measurements signal that decomposition can be entirely attributed to biological processes.
SHANKS, R. Eastward., and J. South. OLSON. 1961. Kickoff-year breakdown of foliage litter in southern Appalachian forests. Science 134(3473):194-195.
OLSON, J. Due south. 1963. Free energy storage and the balance of producers and decomposers in ecological systems. Ecology 44(2):322-331.
In that location may be some photo-oxidation just it is likely to be minor. For a more modern treatment (merely largely based on the model develobet over 30 years agone by Olson) see:
Bosatta, Eastward. and Agren, G.I. 1985. Theoretical assay of decomposition of heterogeneous substrates. Soil Biology and Biochemistry 17:601-610.
Bosatta, E. and Agren, Grand.I. 1995. The ability and reactive continuum models as particular cases of the q-theory of organic matter dynamics. Geochemica et Cosmochimica Acta 59:3833-3835.
Agren, K.I. and Bosatta, E. 1996. Theoretical Ecosystem Ecology. Cambridge University Press.
Q. I understand that atmospheric concentrations of CO 2 are increasing, but when I await at a graph (for example, Keeling's Mauna Loa data), the bend is squiggly. For half of each yr, the concentrations increases, and for the other one-half it decreases. What is the reason for this?
A. The variations inside each year are the result of the annual cycles of photosynthesis and respiration. Photosynthesis, in which plants take up carbon dioxide from the atmosphere and release oxygen, dominates during the warmer part of the year; respiration, by which plants and animals take upwardly oxygen and release carbon dioxide, occurs all the time only dominates during the colder part of the year. Overall, then, carbon dioxide in the atmosphere decreases during the growing season and increases during the rest of the year. Considering the seasons in the northern and southern hemispheres are opposite, carbon dioxide in the temper is increasing in the due north while decreasing in the southward, and vice versa. The magnitude of this bike is strongest nearer the poles and approaches naught towards the Equator, where information technology reverses sign. The cycle is more than pronounced in the northern hemisphere (which has relatively more state mass and terrestrial vegetation) than in the southern hemisphere (which is more dominated by oceans). The Carbon Cycle Group of the NOAA Climate Monitoring and Diagnostics Laboratory (CMDL), has an first-class 3-dimensional illustration of how atmospheric CO ii varies with time year, flavour, and latitude.
Q. How may I perform CO 2 calculations of the carbon dioxide system in seawater?
A. The Programme Developed for CO ii System Calculations (ORNL/CDIAC-105), recently released by Ernie Lewis, Department of Technology, Brookhaven National Laboratory, and Doug Wallace, Abteilung Meereschemie, Institut fuer Meereskunde, was developed to aid calculate inorganic carbon speciation in seawater.
This program, CO2SYS, performs calculations relating parameters of the carbon dioxide organization in seawater and freshwater by using two of the four measurable parameters of the CO 2 organisation [total alkalinity (TA), total inorganic CO two (TCO 2 ), pH, and either fugacity (fCO 2 ) or partial pressure of CO 2 (pCO 2 )] to calculate the other two parameters at a set of input conditions (temperature and pressure) and a gear up of output conditions called by the user.
(Please follow the original ORNL source of the above FAQs for updates.
Back to tiptop
Source: https://www.carboncyclescience.us/what-is-carbon-cycle
0 Response to "what factors contribute to the rate at which carbon moves between the soil and the atmosphere"
Post a Comment