What is a safe way to test for an Acid?
The Acid Test
In this activity, you can:
- find out about acids and bases,
- observe how to make a cabbage water pH indicator and
- learn how to write secret messages.
Please follow whatsoever prophylactic instructions highlighted like this in red.
Acids and bases
What practise lemons and vinegar have in common? They both taste sour on your tongue. This is considering they are acids and acids gustation sour. Bases are the opposite of acids; they ordinarily taste biting and feel soapy. Lots of washing powders are bases ( WARNING: Do non taste these! ). Some other word for a base is an alkali and we say that bases are element of group i compounds.
Some backdrop of acids and bases are:
- acids have a sour taste,
- acids are corrosive,
- acids lose their acidity when they are combined with bases and
- bases feel soapy.
Acids can sound really scary and some of them undoubtedly are, requiring very conscientious handling. Equally a precaution you should be wary of annihilation called an acrid. All the same, in that location are a cracking many acids which are not only harmless to us only are vital to our survival.

Did you know that Vitamin C is another proper noun for a form of ascorbic acid? You'll detect ascorbic acid together with another naturally occurring acid – citric acid – in orange and lemon juice. These are harmless partly considering they are what's known as weak acids.
Acids react with bases and weak acids only really react with very strong bases. The problem with strong acids and strong bases is that they are and so strong that they tin can ever persuade even the weakest of bases or acids to react with them. There usually is something effectually to make full that part since all substances lie somewhere on a calibration between being strong acids and being stiff bases. Virtually natural substances lie in the middle zone where they're neither particularly acidic or basic.
Scientists use something chosen the pH scale to measure how acidic or basic something is. The pH scale measures acerbity in the aforementioned style that a millimetre calibration measures length, for example. A low pH indicates an acidic compound (an acrid), whilst a high pH indicates a basic compound (a base of operations). Here is an illustration of the pH scale from 0 (strongly acidic) to 14 (strongly alkaline) showing how acidic, or basic, some everyday things are: 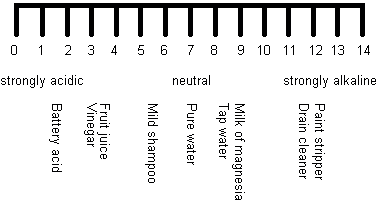
Things that are neither acidic nor basic are neutral and come in the middle of the pH calibration – for example, pure water in the illustration above. Knowing whether something is acidic or bones tin can exist very useful. For instance, wasp stings are alkali metal but bee stings are acidic. So if yous become stung y'all demand to put bicarbonate of soda on a bee sting and vinegar on a wasp sting. Hair conditioner is slightly alkaline every bit shampoo tends to be slightly acidic, so whatever traces of shampoo can be neutralised past the conditioner. What does this tell you about all-in-one shampoo/conditioners?
Back to the top.
How to make an indicator solution
"It's really weird, if you eat a lemon after a ruddy cabbage, you completely change colour"
Special substances called indicators are used to determine whether things are acidic or bones by changing colour. You tin make your own indicator from a red cabbage and find out which things in your kitchen are acidic and bones. To make your indicator solution, you need to:
- Cut the scarlet cabbage upwards, put it into a saucepan and cover with boiling water. Please make sure you are supervised by an adult when handling boiling water.
- Stir it around and so leave it to soak for 15 minutes.
- Using a sieve, split up the liquid from the cabbage pieces.
- Proceed the liquid in an old drinks canteen and characterization it clearly as INDICATOR.
To utilize your indicator solution to test things (the acrid test!):
- Cascade some of the indicator solution into several jars and label them.
- Write CONTROL on one of the jar labels. A control is a re-create of an experiment in which you do nothing. It is used to show that results are due to the examination and non to your equipment, allowing you to do a off-white experiment. This jar will be your control, so you won't be calculation anything to it – it's at that place so y'all tin compare it to the others.
- To the other jars of indicator solution, add a few drops, or bits, of the things you want to test. For example: lemon juice, cola, vinegar, a boiled sweet, washing upward liquid, detergents, bicarbonate of soda, shampoo and conditioner.
- Observe any color changes in the jars, compared with the control. You lot can use this cabbage indicator pH scale to make up one's mind the acerbity:
pH 2 4 6 8 10 12 Colour carmine imperial violet blue blue green green
Back to the top.
Invisible ink
You lot can even utilize the power of acrid and base chemistry to write clandestine messages! Yous tin can write a message in invisible ink by doing this:
- Add together 1 tablespoon of bicarbonate of soda (blistering soda) to a mug of h2o.
- Dip a cotton wool-wool bud into the mixture and use it like a pen to write your message onto a slice of paper. Handy hint: try non to put too much liquid onto the cotton wool bud!
- When the paper is completely dry, rub a blood-red grape over the message. Handy hint: using a hair dryer speeds things upwardly.
Can you work out what's going on? Of the bicarbonate of soda and the crimson grape, which do you think is acidic and which element of group i?
This activity was used by united states for Science Calendar week 2003 and at the BAYSday 'Hands-on Science' consequence held at Majestic Higher, London in March 2003.
Source: https://www.york.ac.uk/res/sots/activities/acidtest.htm
0 Response to "What is a safe way to test for an Acid?"
Post a Comment